Working in a laboratory is inseparable from the risks that might occur. The laboratory is a place with great potential risks with various sources and types of risks. Risks that can occur include physical hazards, chemical hazards, biological material hazards, and other hazards. When viewed from the potential hazards that occur in the laboratory, it is important to carry out a risk assessment so that the laboratory director can manage and reduce risks for employees in the laboratory. Prevention can be done by creating and developing safety procedures and actions that must be taken in case of exposure, contamination or injury. When conducting an assessment, consider a wide range of safety, health and environmental hazards, from machine safety to physical hazards to chemical and biological exposure.
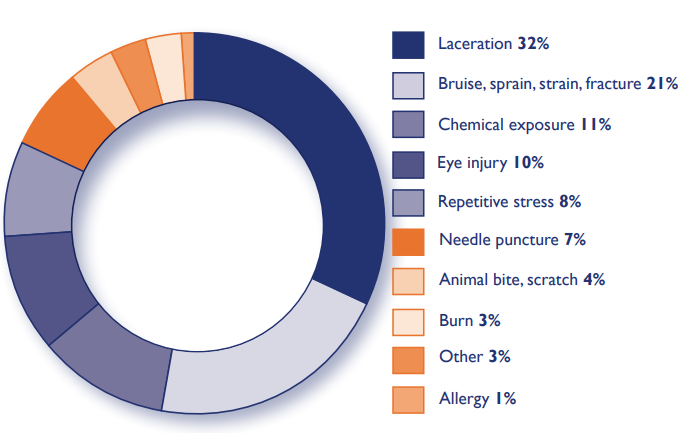
Results of a study identifying physical hazards experienced by laboratory employees by the Howard Hughes Medical Institute Office of Laboratory Safety. (source: Lab Quality Management System Handbook)
Referring to the data in the Lab Quality Management System (LQMS) Handbook where a study on the identification of physical hazards experienced by laboratory employees was carried out by the Howard Hughes Medical Institute Office of Laboratory Safety, the potential hazards and how much they occur can be seen in the following diagram. Lacerations are the most common hazard for laboratory employees, followed by bruises, sprains, strains and fractures. Instruments in the laboratory are dominated by glass which is prone to shattering, needles and other sharp objects that can injure the skin must be handled and disposed of properly. In addition, the risk of fire and electrical hazards also often occurs, so that electricity or objects that have the potential to cause a fire must be managed as well as possible and facilitated by fire extinguishing equipment.
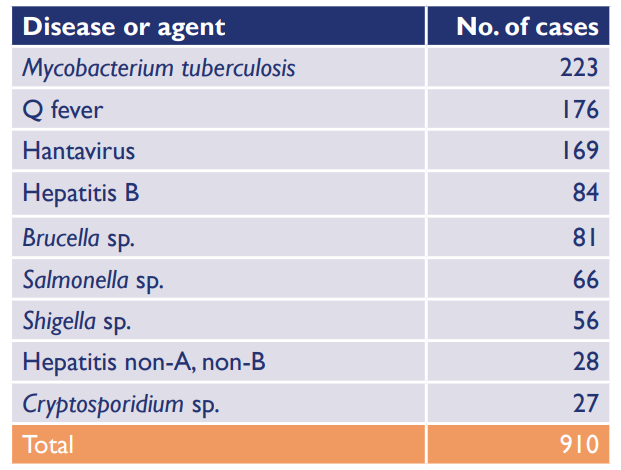
Laboratory reports in the United States of the most common infecting biological agents in laboratories. (source: Lab Quality Management System Handbook)
In addition to physical hazards, exposure to toxic chemicals poses a real threat to health and safety. There are more than 400 chemicals that have the potential to injure laboratory employees, so they are required to store, use and dispose of chemicals according to safety procedures. There are three main routes by chemicals enter the body: inhalation, absorption through the skin, and accidental ingestion. There is treatment if an accident occurs in the laboratory with a description; If inhaled, get fresh air; if exposed to skin or eyes, remove all clothing and rinse under running water for at least 15 minutes; and if swallowed, drink at least two glasses and don’t induce vomiting.
Other risks are caused by biological hazards, including viruses, fungi, bacteria, transgenic animals, experimental animals, insects, toxins, and allergens. The following table shows the most frequently reported laboratory infections in the United States.
In preventing risks that may occur, the laboratory needs to pay attention to the precautions listed in the material safety data sheet (MSDS). MSDS is a technical bulletin that provides detailed information on hazards and precautions to ensure the chemicals they use are handled and stored safely.
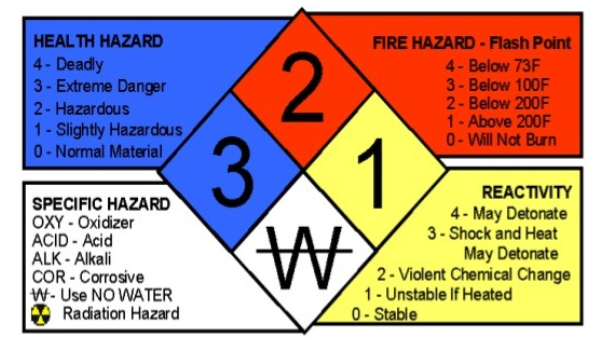
National Fire Protection Association (NFPA) system for classification of hazards
MSDS provide:
- product information;
- fire and explosion prevention;
- toxicology;
- health effects;
- recommended PPE;
- storage recommendations;
- leaks and spills—action recommended;
- waste disposal recommendations; and
- first aid.
Bibliography:
National Research Council (US) Committee on Prudent Practices in the Laboratory. (2011).Prudent Practices in the Laboratory: Handling and Management of Chemical Hazards: Updated Version.Washington (DC): National Academies Press (US).Accessed at: https://www.ncbi.nlm.nih.gov/books/NBK55880/
Eko dkk.(2020).Panduan Pengelolaan Mutu Laboratorium dalam Jejaring Laboratorium One Health.Bogor:IPB Press.Accessed at: https://onehealthlab.net/blog/13139/?lang=id
World Health Organization.(2011).Lab Quality Management System.France: WHO Lyon Office.Accessed at: https://www.who.int/publications/i/item/9789241548274


Leave a Reply